
In June, we published an FAQ for authors and librarians to give some guidance on how they might respond to NIH’s accelerated implementation of its public access plan, which requires immediate availability of sponsored research articles upon publication. Our FAQ from June is still good advice, but since then both the NIH and several publishers have updated their guidance and so we are giving some additional information about the latest here.
The full set of NIH materials regarding its new public access policy are available at the following links:
- New NIH Public Access Policy Effective July 1, 2025
- NIH Grants & Funding–Public Access Policy– Frequently Asked Questions (FAQs) (updated on July 1, 2025)
- NIH NOT-OD-25-047 (2024 NIH Public Access Policy)
- NIH Notice NOT-OD-25-049 (Supplemental Guidance to the 2024 NIH Public Access Policy: Government Use License and Rights)
- NIH Notice NOT-OD-25-101 (Revision: Notice of Updated Effective Date for the 2024 NIH Public Access Policy)
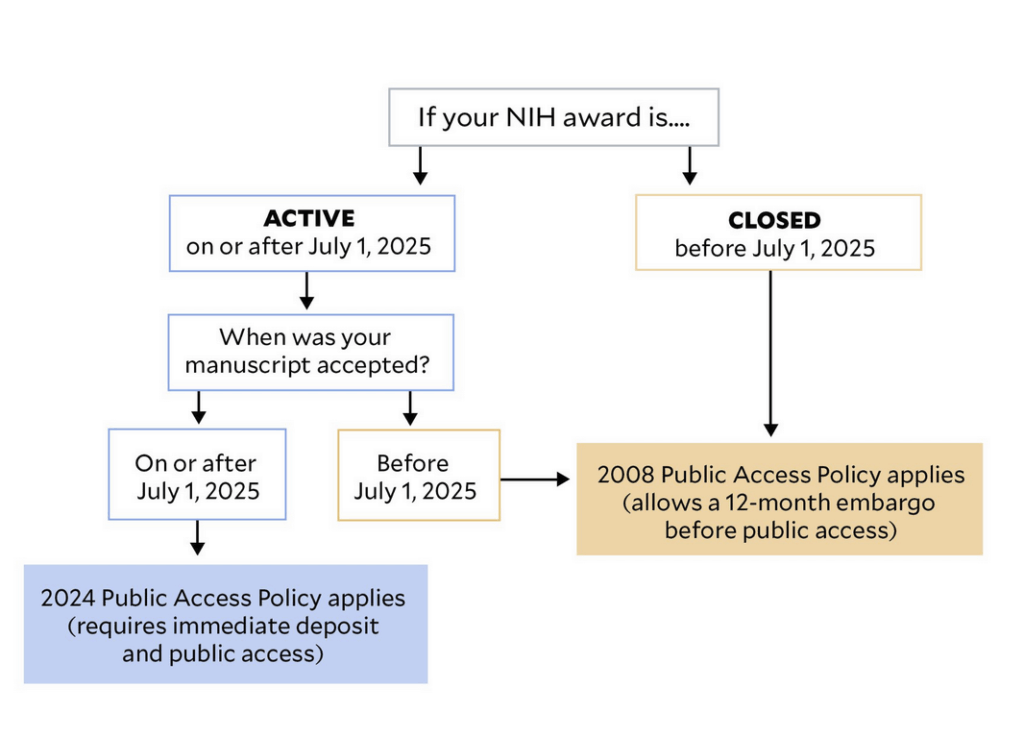
Exactly which articles are subject to the new policy?
We’ve heard numerous questions about the timing of how this new public access policy applies, with authors and libraries expressing particular concern about articles that had already been submitted but not yet accepted by journals when this policy became effective on July 1. This has been a particular worry for authors who had submitted to journals without considering whether their publication policies complied with zero-embargo public access requirements.
Early in June, we explained that the NIH policy initially indicated that “author Accepted Manuscripts […] with acceptance dates on or after July 1, 2025, are subject to the Policy,” full stop. Since then, the NIH has clarified that the new public access policy actually doesn’t apply if your grant has closed prior to July 1, even if your article was accepted for publication after July 1: “For awards that were closed prior to July 1, 2025, authors with any subsequent Author Accepted Manuscripts resulting from and citing the closed NIH award are expected to continue to comply with the 2008 Public Access Policy, which allows for an up-to 12-month embargo.”
For those on university campuses charged with explaining the new policy, this injects some complexity in determining which articles are subject to the policy, but it also means that for at least some NIH-funded authors who hadn’t considered their journal’s stance on zero-embargo deposit or open access, they aren’t in the bind that they otherwise might have been.
To help make this especially clear, we are thankful to Dana Haugh, Harvey Cushing/John Hay Whitney Medical Library, Yale, who created this chart to explain:
Is the ACS Article Development Charge an “allowable cost”?
Another recurring question we’ve heard is about which costs are allowable under NIH grants, particularly in light of some of the variety of new fees that some publishers have invented to allow for zero–embargo deposit but that fall short of full, gold open access. There has been lots of conversation about the American Chemical Society’s “Article Development Charge” – a $2500 charge that applies when authors seek to publish in a hybrid-OA ACS journal, the article includes funder-recommended or required language requiring zero-embargo, and the author isn’t otherwise covered by an institutional open access agreement.
Whether the ACS article development charge is an allowable cost under NIH guidance is a matter of debate. ACS, for its part, characterizes the article development charge as “not a submission fee nor a deposition fee,” and also tells authors that “[t]he ADC may be an allowable publication cost under the terms of many federal and international research grants, meaning authors may be able pay it using grant funding.” But ACS goes on to warn authors that authors “should confirm with their funder before choosing this option.”
The NIH has yet to publicly comment specifically on whether the ACS’s Article Development Charge is an allowable cost. However, in its “Supplemental Guidance to the 2024 NIH Public Access Policy: Publication Costs the NIH places several restrictions on publication costs. One is that “Journal or publisher fees that arise during the course of the publication process for the sole purpose of submitting the Author Accepted Manuscript to PubMed Central are not allowable costs.” ACS’s new fee presumably does not run afoul of this restriction because it is not actually for PMC submission, as ACS makes clear.
But a separate NIH publication cost restriction may be a bigger problem. The same guidance states: “costs for publishing services that are charged differentially because an Author Accepted Manuscript is subject to the NIH Public Access Policy or the work is the result of NIH funding are unallowable because charges must be levied impartially on all items published by the journal, whether or not under a federal award (GPS 7.9.1).” ACS doesn’t come out and say “we are treating NIH grantees differently and they must pay this fee.” But, reading the ACS page explaining the purpose of the ADC, along with the timing of its implementation and its requirements, the policy in effect treats those NIH papers differently than other papers not subject to a federal award. If I were an author under an NIH grant, I would at least double-check before submitting an ACS article development charge
Perhaps in recognition of this murkiness, ACS announced on July 7 that it would be giving NIH-funded corresponding authors a temporary reprieve from its newly invented article development charge. ACS’s announcement in full explains:
To provide time for institutions, funders, and ACS Publications to identify long term solutions—ACS Publications is sponsoring zero-embargo green open access for NIH-funded corresponding authors who submit to our hybrid journals through July and August 2025.
This program will allow eligible authors to comply with the adjusted NIH Public Access Policy at no cost to them. This includes manuscripts already submitted and under editorial review, and all eligible submissions up to and including August 31, 2025. Eligible authors will be prompted after acceptance with the option to retain copyright of the accepted manuscript. Selecting yes will ensure they can deposit the accepted manuscript in accordance with NIH guidelines.
Have any of the other large scientific publishers changed their stance?
While the ACS publishing options have generated lots of discussion, several of the other major publishers have adopted a straightforward approach: they have mostly doubled down on their insistence that the only way to publish with them is by paying for open access, and have pulled back on automatically depositing articles to PubMed Central for authors who follow a subscription publishing route.
Springer Nature now states that “Publishing via the subscription route is not a viable option: Choosing the subscription publication route in a Springer Nature journal conflicts with immediate public access policies, such as NIH’s policy. Authors will therefore need to opt for gold OA in order to comply with the NIH’s policy. For more information, please see our FAQ below on complying with immediate access requirements.”
Elsevier has deleted its page explaining how it would deposit articles to the PMC (archived copy from 5/18/25) and now simply refers authors to its Journal Article Sharing page, which indicates that the only pathway to zero-embargo sharing it to purchase Elsevier’s Gold OA option.
Wiley has been confusing. Wiley’s policy, prior to the NIH accelerated public access policy, stated that Wiley would support NIH deposit to PMC: “Wiley will deposit the accepted version of NIH funded articles to PubMed Central (PMC) on acceptance by the journal. Once Wiley has posted the files, authors will receive an email to approve the upload for display in PMC. This approval is a requirement of their grant/affiliation. This accepted version will be made publicly available 12 months after publication.”(archived 5/22/25)
Then, Wiley revised its guidance (archived 7/2/25) to say that Wiley would no longer deposit subscription articles to PMC on authors’ behalf. Wiley at the same time reminded authors that they must comply with the Wiley self archiving policy and indicated that authors who deposited on their own would be in violation of their publishing agreement: “Our previous PMC deposit policy required a deposit process for NIH-funded, non-OA articles with a 12-month embargo. Because of our new policy, this process will no longer be in effect. Authors who deposit before the end of the embargo period for non-OA articles will be in viloation [sic] of their license agreement.” (emphasis added)
But then, Wiley updated its guidance again. In the current live version of this same guidance, Wiley entirely removed the line about authors being in violation of their publishing agreement if they deposit themselves before the end of the embargo period.
We find the whole thing especially confusing because Wiley has not updated their standard Copyright Transfer Agreement, which as we noted in our June FAQ, states that “In the case of a Contribution prepared under U.S. Government contract or grant, the U.S. Government may reproduce, without charge, all or portions of the Contribution and may authorize others to do so, for official U.S. Government purposes only, if the U.S. Government contract or grant so requires. This is precisely what the NIH and other agencies are doing in their exercise of the federal purpose license, so it seems to us that a straightforward reading of this clause means that it’s perfectly acceptable for authors to work with the NIH to deposit in furtherance of its official government purpose.
It’s worth a reminder that not all publishers have sought to clamp down on the deposit of manuscripts to PMC. Other publishers such as AAAS, Sage, BMJ and many others allow for zero embargo deposit of Author Accepted Manuscripts in compliance with NIH’s public access policy. The NIH FAQ reminds authors that “Journals with an active agreement to deposit all articles can be found in the PMC Journal List, which is available at https://pmc.ncbi.nlm.nih.gov/journals/. NIH recommends reviewing the specific journal record to confirm that the Release Delay is 0 months (Immediate Release) and the Agreement Status is Active. “
What does the NIH announcement about capping publication costs mean for authors now?
Finally, only a few days after the effective date of the new NIH public access policy, the NIH announced another major change: in the new fiscal year (which starts October 1) NIH will introduce a cap on allowable publication costs. The timing was unfortunately confusing–we’ve heard from some people already asking if this cap was in effect on July 1 (it is not). So for now, it has no effect on authors. However, given the clear indication that NIH will begin implementing such caps, we think it’s good advice for authors to think carefully about what fees they might incur for papers submitted now and that may be accepted or published in FY2026. As we expressed here, the devil will be in the details in determining how low the cap will be. The NIH announcement gives few hints about what that number will be, but the NIH announcement is incredulous that “some major publishers charge as much as $13,000” (Nature charges $12,690), so certainly authors should be careful with submissions to journals with those higher fees.
Discover more from Authors Alliance
Subscribe to get the latest posts sent to your email.

Pingback: NIH Public Access Policy: Implementation issues – Inside Science Resources
Pingback: Hinweise zur neuen Public Access Policy des National Institutes of Health in den USA – Archivalia
Pingback: Day in Review (July 21–July 24) — Association of Research Libraries
Pingback: NIH APC Cap Survival Blueprint: What You Need to Know Now! -
Pingback: …so what exactly is going on between publishers and the NIH? - ACRLog